RHA Filler

Meet RHA Filler
The weightless lipstick line treatment.
RHA Redensity™ is dynamically different and designed to be resilient enough to adapt to your dynamic facial movements for a look that’s beautiful at rest and flawless in motion. Experience the only FDA-approved hyaluronic acid filler made to smooth dynamic lipstick lines for natural-looking results. To learn more about the RHA® Collection, visit RHACollection.com.

BEAUTIFUL AT REST
FLAWLESS IN MOTION
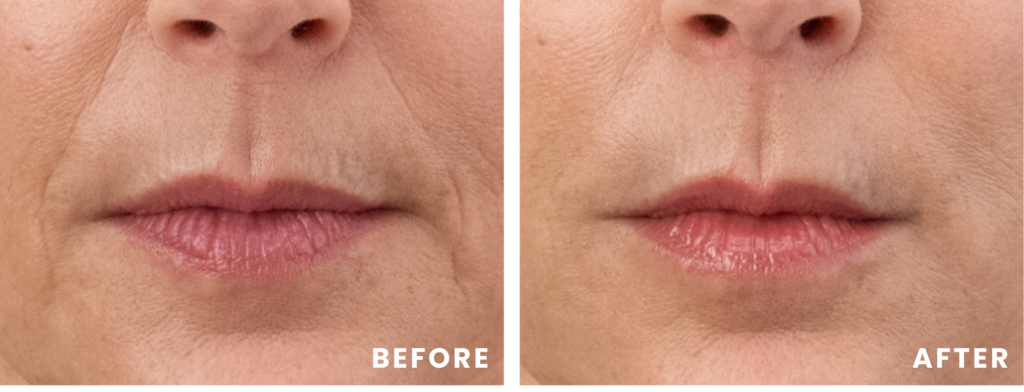
Cathy
Cathy’s lipstick lines were improved with 0.7 mL of RHA Redensity™, and her dynamic wrinkles and folds in her lower face were improved with 1.5 mL of RHA® 2 and 1 mL of RHA® 3. Images captured before and two weeks after treatment. Results may vary.


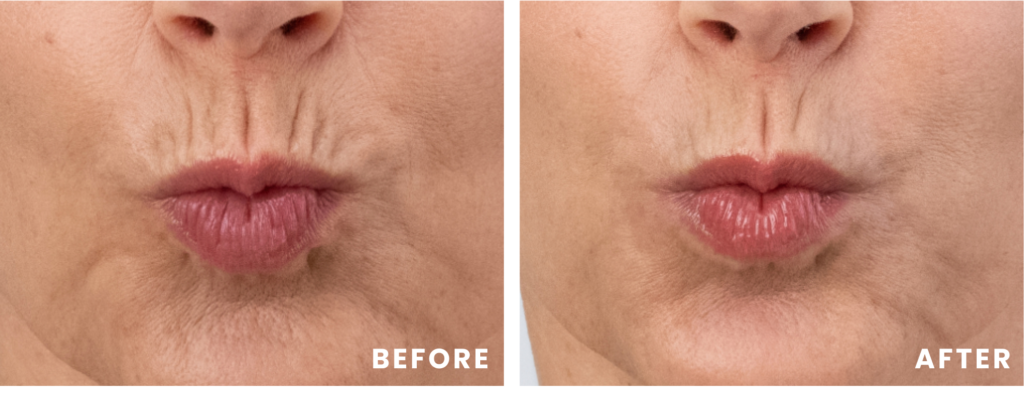
Georgia
Georgia’s lipstick lines were improved with 0.9 mL of RHA Redensity™, and her dynamic wrinkles and folds in her lower face were improved with 2 mL of RHA® 2 and 1.8 mL of RHA® 4. Images captured before and two weeks after treatment. Results may vary.
RHA Redensity™
Approved Uses
The RHA® Collection of resilient hyaluronic acid (HA) fillers includes RHA Redensity, RHA® 2, RHA® 3 and RHA® 4.
RHA Redensity™ is for injection into the facial tissue for the correction of moderate to severe dynamic perioral rhytids; and RHA® 2, RHA® 3 and RHA® 4
are for the correction of moderate to severe dynamic facial wrinkles and folds, such as nasolabial folds, in adults 22 or older.
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive any RHA® injectable gel formulation?
Do not receive if you have a history of multiple severe allergies or severe allergic reactions; if you are allergic to lidocaine or gram-positive bacterial
proteins; or if you have a bleeding disorder.
What precautions should I discuss with my doctor?
- Tell your doctor if you are pregnant or breastfeeding as the safety of these products for use during pregnancy or while breastfeeding has not
been studied - Tell your doctor if you have a history of excessive scarring, keloid formations or pigmentation disorders, as use of these products may result in
additional scars or changes in pigmentation - Tell your doctor if you are planning laser treatments or a chemical peel, as there is a possible risk of inflammation at the treatment site if these
procedures are performed after treatment - Tell your doctor if you are on immunosuppressive therapy used to decrease your immune response, as use of these products may result in an increased risk of infection
- Tell your doctor if you are using medications that can prolong bleeding, such as aspirin, ibuprofen, or other blood thinners, as this may increase bruising or bleeding at the injection site
- The safety and effectiveness of RHA® fillers in areas other than those indicated have not been established in U.S. clinical studied
- Patients who experience skin injury near the site of injection with this product may be at a higher risk for side effects
- Minimize strenuous exercise, exposure to extensive sun or heat, and alcoholic beverages within the first 24 hours following treatment
What are possible side effects?
The most commonly reported side effects included injection-site redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching.
One of the risks with using these products is unintentional injection into a blood vessel, and while rare, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring.
Delayed-onset inflammation near the site of dermal filler injections is one of the known side effects associated with dermal fillers. Cases of delayed-onset inflammation have been reported to occur at the treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. Typically, the reported inflammation was responsive to being treated or resolved on its own.
To report a side effect with any RHA® product, please call Revance at (877) 373-8669. Please visit RHACollection.com or talk to your doctor for more information.
Available by prescription only.
RHA-P-002435.2
